Blackbody Radiation
Quick Exam Notes
- Concept: Blackbody is an ideal object that absorbs and emits all incident radiation.
- Rayleigh–Jeans Law: \( E(\lambda, T) \propto \frac{1}{\lambda^4} \) — valid only at long wavelengths, fails at short wavelengths (ultraviolet catastrophe).
- Wien’s Displacement Law: \( \lambda_{max} T = \text{constant} \) → peak wavelength shifts inversely with temperature.
- Planck’s Law: \( E = \frac{8 \pi h c}{\lambda^5} \cdot \frac{1}{e^{\frac{hc}{\lambda kT}} - 1} \) — explains full spectrum using quantized energy.
- Key Point: Classical theory failed, but Planck’s quantization solved blackbody spectrum problem → foundation of quantum mechanics.
- Applications: Astrophysics, thermal radiation, climate studies, infrared imaging, incandescent lamps.
- Scroll to the end of the page to explore the Interactive Visualization demo of blackbody radiation spectrum.
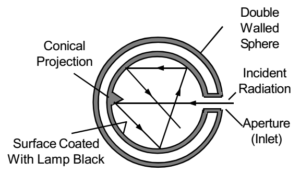
A blackbody is a body which absorbs radiations of all wavelengths which incident on it. Blackbody neither reflects nor transmits any of the incident radiation, and therefore, appears black. When any radiation enters the blackbody region through the opening, it suffers multiple reflection inside the sphere and is finally absorbed. When the walls of such a cavity are heated to temperature T, it emits the radiations which fill the cavity and it come out through the opening of cavity. These radations are known as blackbody radiations, which are the characteristic of its temperature.
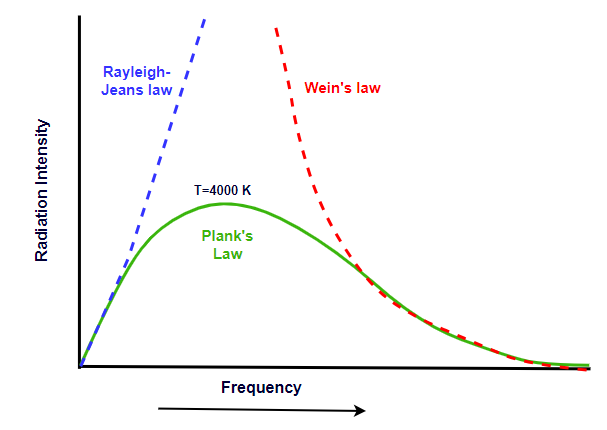
Rayleigh-Jeans law of black body radiation
According to Rayleigh-Jeans law, the energy distribution in the thermal spectrum is given by
\[ E_\lambda = \frac{8\pi kT}{\lambda^4} \]The Rayleigh-Jeans law holds good in the region of shorter frequencies but not for longer frequencies.
Wien’s displacement law of black body radiation
According to Wiens displacement law, in the energy spectrum of a black body, the product of the wavelength corresponding to maximum energy ( \( \lambda_m \)) and absolute temperature is constant. i.e.,
\[ \lambda_m T = constant \]The Wien’s law holds good in the region of longer frequencies but not for shorter frequencies.
Plank’s law of black body radation
According to Plank's theory energy is emitted in the form of packets or quanta called photons and energy of photons is given by \( E=nhv \) where n=1,2,3 etc.
The spectral radiance (energy emitted per unit area, per unit solid angle, per unit frequency) of electromagnetic radiation from a black body at a given temperaturein terms of freuqency is given by
\[ E_\lambda = \frac{8 \pi h c}{\lambda^5} \cdot \frac{1}{e^{\frac{hc}{\lambda kT}} - 1} \]where h is Plank’s constant, c is speed of the light and T is the temperature of the enclosure.
Plank’s law successfully anticipated black body radiation at higher and lower frequencies
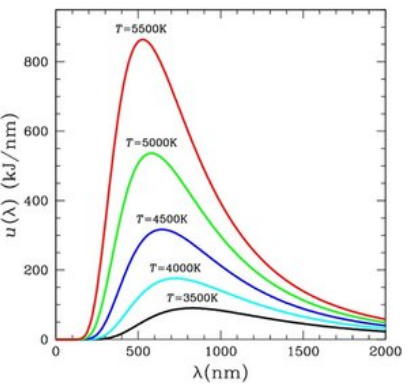
Black body radiation spectrum (Characteristics of a blackbody)
- At a given temperature, the energy is not uniformly distributed in the radiation spectrum of the blackbody.
- At a given temperature, the intensity of radiation increases in wavelength and at a particular wavelength, its value is maximum. With further increase in wavelength, the intensity of radiation decreases.
- With the increase in temperature, λm decreases, where λm is the wavelength at which the maximum emission of energy. takes place. The points on the dotted line represent λm at various temperatures.
- There is increase in energy emission with the increase in temperature corresponding to all the wavelengths.
- The area under curve gives the total energy emitted for the complete spectrum at a particular temperature. With increase in temperature, this area increases. It is also observed that the area is directly proportional to the fourth power of the temperature of the blackbody, i.e., E ∝ T4
Summary
Blackbody radiation refers to the thermal electromagnetic radiation emitted by an idealized object that absorbs all incident energy. Classical theories like the Rayleigh–Jeans law failed to explain the observed spectrum (ultraviolet catastrophe). Wien’s displacement law describes the shift of peak wavelength with temperature, while Planck’s law introduced quantized energy levels, providing a complete explanation of the blackbody spectrum. This concept laid the foundation of quantum mechanics and is essential in astrophysics, thermal physics, and modern technology.
MCQs on Blackbody Radiation
-
Blackbody radiation is the radiation emitted by:
- a) A perfect reflector
- b) An ideal absorber
- c) A transparent body
- d) A polished metal surface
Answer
b) An ideal absorber
-
The failure of the Rayleigh–Jeans law at short wavelengths is known as:
- a) Infrared catastrophe
- b) Microwave breakdown
- c) Ultraviolet catastrophe
- d) Quantum limit
Answer
c) Ultraviolet catastrophe
-
Wien’s displacement law states:
- a) λmax T = constant
- b) E = mc²
- c) λ T² = constant
- d) Energy ∝ T⁴
Answer
a) λmax T = constant
-
Planck’s law of blackbody radiation introduced the concept of:
- a) Continuous energy distribution
- b) Energy quantization
- c) Zero-point energy
- d) Infinite energy levels
Answer
b) Energy quantization
-
Which of the following is an application of blackbody radiation?
- a) Study of stellar temperatures
- b) Infrared imaging
- c) Climate science and radiation balance
- d) All of the above
Answer
d) All of the above