Surface area to volume ratio
Nanomaterials have a relatively larger surface area, when compared to the same volume. Let us consider a sphere of radius ‘r’.
Surface area = 4pir2
volume = 4/3 pi r2
Hence, the surface to volume ratio for a sphere will be
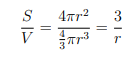
The smaller the radius/size of any material, higher the surface area. Thus nanoparticles have a much greater surface area as compared with larger particles. It makes materials more chemically reactive. This affects their strength or electrical properties hence they are used mainly in sensors.
A characterization parameter for the effectiveness of a nanoparticle is the ratio of its surface area to its volume. Explain with example of a cube.
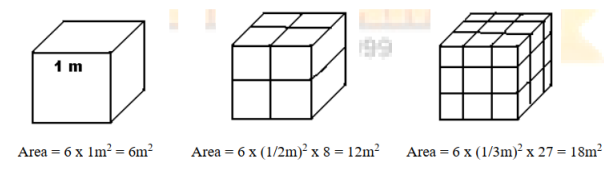
Let us consider an example. For a one cubic volume shown in figure, the surface area is 6m2. When it is divided into eight pieces its surface area becomes 12m2. When the same volume is divided into 27 pieces its surface area becomes 18m2. Thus when given volume is divided into smaller pieces, the surface area increases. Hence as particle size decreases, a greater proportion of atoms are found at the surface compared to those inside. Thus nanoparticles have a much greater surface area compared with larger particles.