Extrinsic Semiconductor
Extrinsic semiconductor are obtained by adding small amount of impurities in intrinsic semiconductor. Addition of these impurities preferentially increase the carriers of any one type (either e- or h+). These impurities are called as dopants which are controlled very precisely during the manufacturing process and are also used in very small amount so that the original silicon lattice will not be disturbed.
Depending the type of dopants used, extrinsic semiconductors can be of two type
- p-type semiconductor
- n-type semiconductor
1) n-type semiconductor
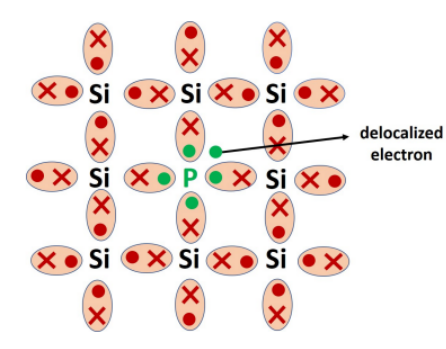
When intrinsic semiconductors are doped with pentavalent material from Group V (Eg:phosphorous, arsenic, antimony) of periodic table, we get n-type semiconductor.Phosphorous has 5 electrons, four of these electrons can easily form a bond with silicon and that lives behind one extra electron. Now this extra electron that is available can be delocalized and can be made available for conduction. So by adding phosphorous atoms, for each phosphorous atom we have created one extra electron in the system.
2) p-type semiconductor
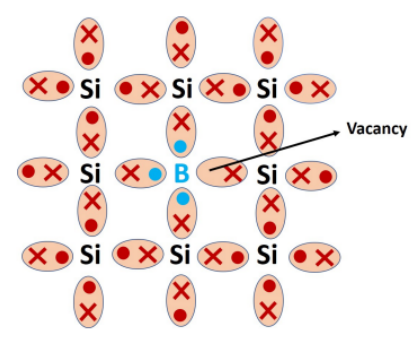
When intrinsic semiconductors are doped with Group III element (Eg: boron, aluminum, gallium) of periodic table, p-type semiconductors are obtained. When Boron (B) is added in a very small amount in pure Silicon, three electron of Boron form 3 bonds with 3 Si atoms shown in Fig 4. But this silicon atom has one extra electron. In order to form the bond with this silicon atom, an electron from the valence band is excited to the boron level leaving a hole behind.
Examples of extrinsic semiconductors are silicon and germanium made of impurities such as As, P, Bi, Sb, In, B, Al, etc.
MCQs on Extrinsic Semiconductors
-
An extrinsic semiconductor is formed by:
- a) Heating pure silicon
- b) Adding controlled impurities (doping)
- c) Removing electrons
- d) Cooling intrinsic semiconductors
Answer
b) Adding controlled impurities (doping)
-
In an n-type semiconductor, the majority carriers are:
- a) Holes
- b) Electrons
- c) Protons
- d) Neutrons
Answer
b) Electrons
-
Which of the following is a typical dopant for creating a p-type semiconductor?
- a) Phosphorus (P)
- b) Boron (B)
- c) Arsenic (As)
- d) Antimony (Sb)
Answer
b) Boron (B)
-
In an extrinsic semiconductor, the conductivity:
- a) Increases with doping
- b) Decreases with doping
- c) Remains the same as intrinsic
- d) Becomes zero at high doping
Answer
a) Increases with doping
-
The donor energy levels in an n-type semiconductor are located:
- a) Just above the valence band
- b) Within the valence band
- c) Just below the conduction band
- d) At mid-gap
Answer
c) Just below the conduction band